What is a clinical trial?
Learn about clinical trials, from their early origins to how they're run today
What is a clinical trial?
A clinical trial (also known as a clinical study) is a type of medical research that human volunteers take part in. Clinical trials help us answer questions about a drug, device, or diagnostic test, such as:
Thousands of people take part in clinical trials, globally, every year. Without them, medical progress would simply not be possible.
The history of clinical trials
What many people don’t realize is that clinical research has been around for many years – centuries in fact! So, where did it all begin?
The first scientific experiments
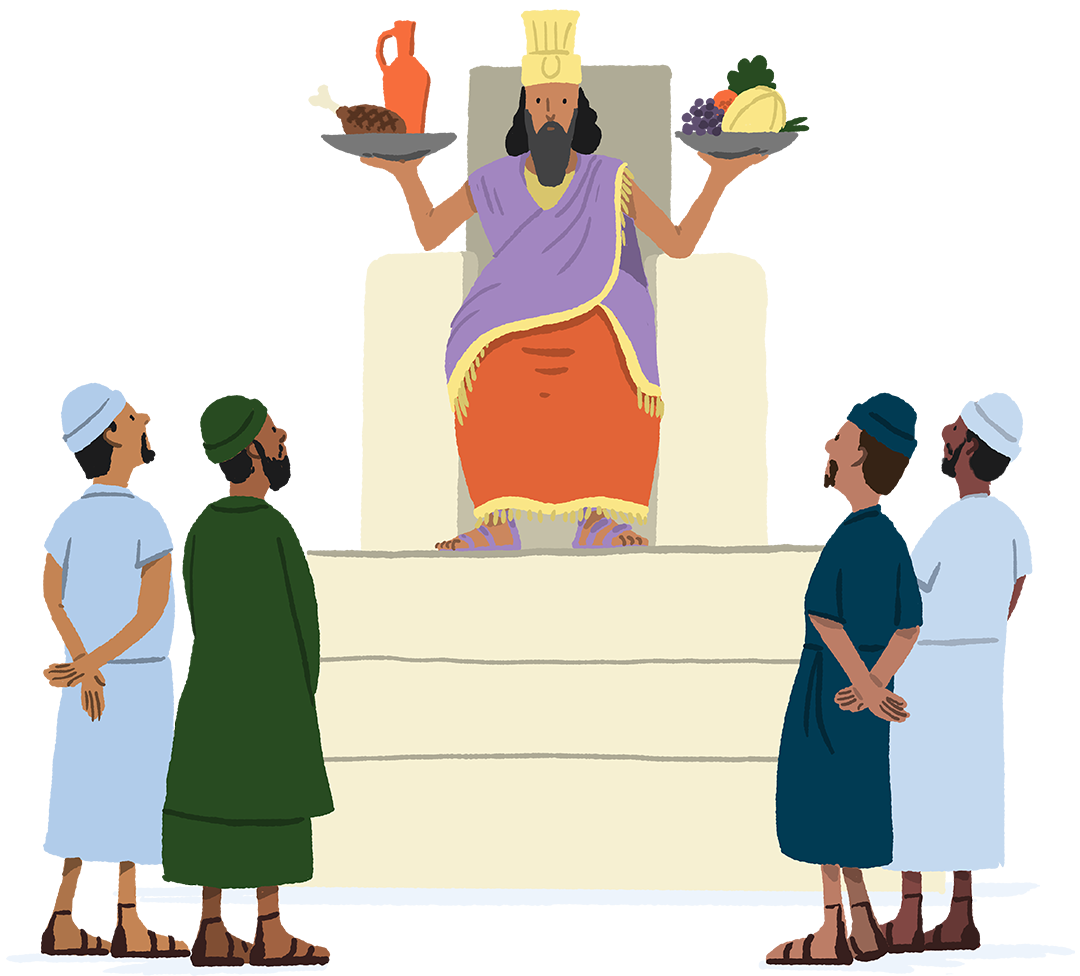
The first documented experiment in people was conducted by King Nebuchadnezzar. He prescribed a diet of meat and wine for his subjects. But the vegetarians objected and ate their normal diet for ten days, while the others ate the investigational diet. The vegetarians looked healthier to the king, so he allowed them to continue their diet.
Ambroise Paré

When Ambroise Paré – a surgeon responsible for looking after soldiers – ran out of boiling oil solution (commonly used as a treatment for wounds) he had to think of a new treatment option. He experimented with a combination of egg yolks, rose oil, and turpentine, which – to his surprise – worked better than oil on its own!
James Lind
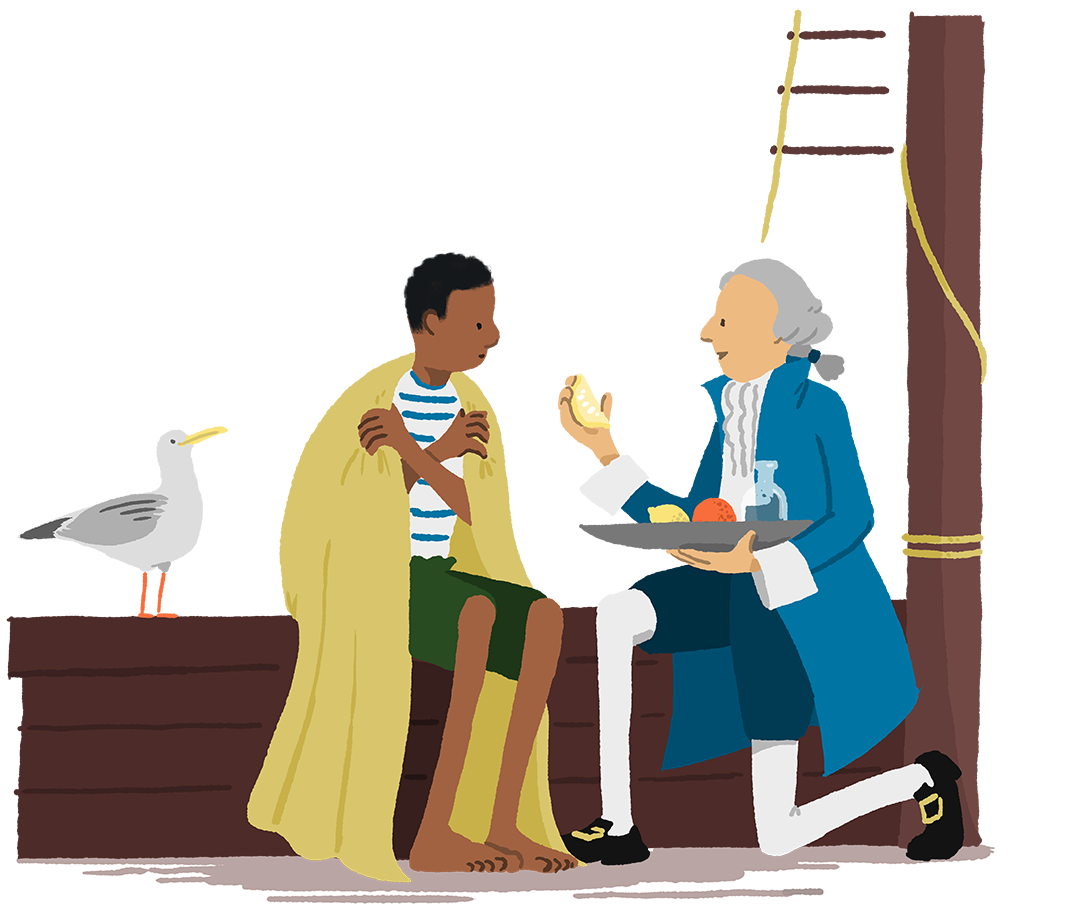
In the 18th century, scurvy – caused by Vitamin C deficiency during long sea voyages – was very serious. When his crew became ill, naval surgeon James Lind trialed potential treatments, from seawater to oranges and lemons. He found that patients who ate citrus fruits recovered. This still informs our dietary guidelines today.
Placebo

The word placebo first appeared in medical literature in the early 19th century, used to describe ‘any medicine more to please than benefit the patient.’ In 1863, U.S. doctor Austin Flint carried out the first medical experiment comparing a placebo (a 'dummy' treatment) with an active one. Placebos are still used in clinical trials today to see whether any improvements in those taking part are the result of the investigational drug or something else.
First double-blind, controlled clinical trial
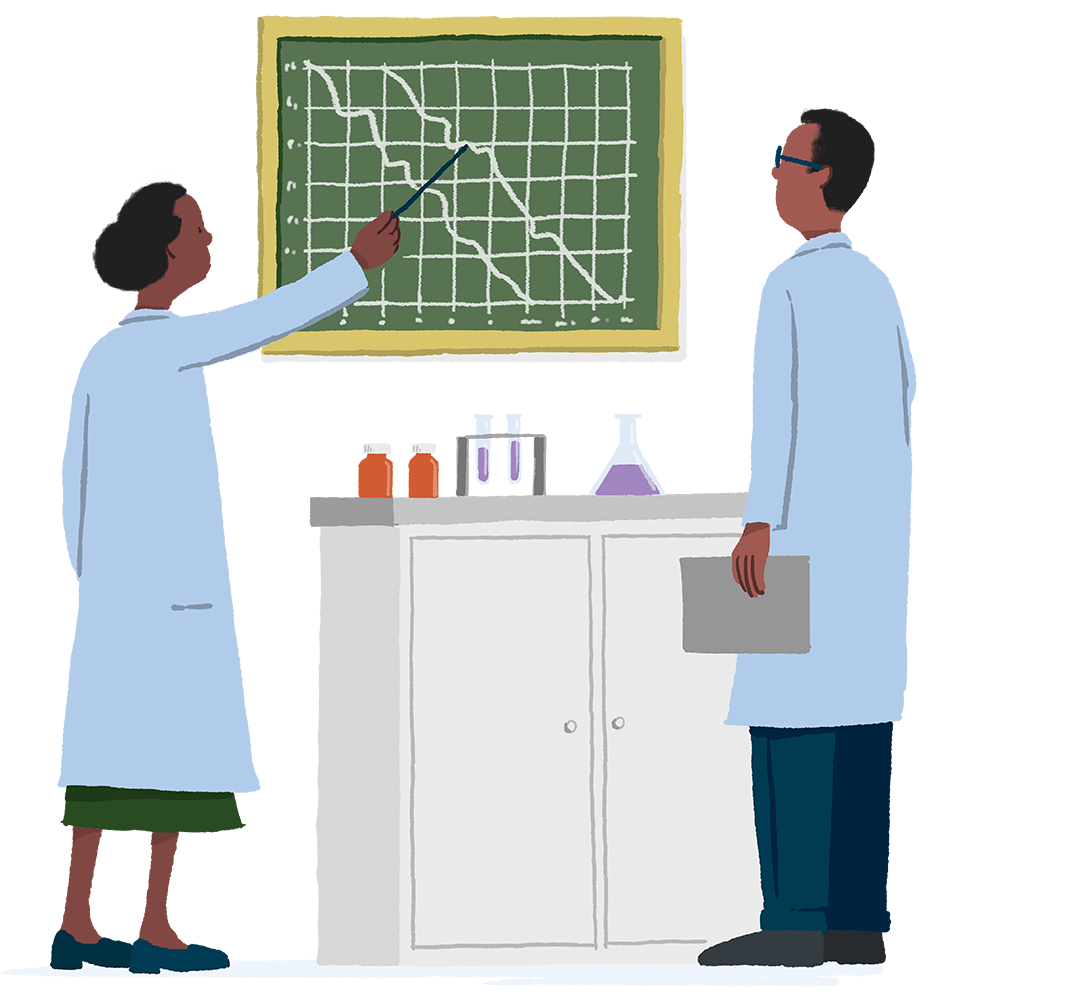
During World War II, an investigational drug – patulin – was tested for treating the common cold and compared with placebo. Unfortunately, patulin didn’t work, but the research project still goes down in history as the first well-controlled trial.
Informed consent
Human experimentation, both in the form of the Tuskegee Study and under the Nazi regime, horrified the world and called for new medical research laws. In 1947, the Nuremberg code was created to ensure participants gave consent before taking part in any medical research. This was further regulated in 1962 with the Kaefavuver-Harris amendments in the United States and the Helsinki Declaration in 1964.
To learn more about medical injustices like the Tuskegee study, see our interactive participant rights timeline below.
Where are we now?
Clinical trials provide valuable information to improve our understanding of different conditions. Safety measures and regulations have been put in place to make sure unethical medical injustices are never repeated in today's clinical trials.
You have the opportunity to contribute to the health of your community
Diversity in clinical trials not only helps fight health inequalities, it can lead to more knowledge about diseases and potential treatments for all. That’s why today, we are trying to have better representation in clinical trials.
When you take part in a clinical trial, you’re supporting your family, friends, and entire community. Your participation provides unique and important information that can help researchers learn more about diseases and potential treatment options.
The way a drug works in the body can vary for different groups of people. This means accurate representation of people in clinical trials – across age, gender, race, ethnicity, and socio-economic level – not only helps fight health inequalities and rebuild trust, it can lead to more knowledge about diseases, and potential treatments for all.
Who's involved in a clinical trial?
When learning about a clinical trial, you might hear about the many people involved. But who exactly are these people, and what do they do?
The sponsor funds, initiates and manages a trial. They can be a company (such as pharmaceutical or biotech), non-profit institution, doctor, or government organization. We (Biogen, a biotech company) are a clinical trial sponsor.
Every country has a regulatory authority with its own laws for running a trial. Each authority must review and approve the protocol before a trial can begin. They must also make sure that the trial follows national regulations.
An independent team of people who review trials to protect the rights and welfare of the people who take part.
Also known as a Data and Safety Monitoring Board (DSMB), the Data Monitoring Committee (DMC) monitors the reliability of data collected in trials to protect participant safety. This small group of experts (such as statisticians and disease experts) are independent of the trial sponsor and review this data without bias.
The principal investigator supervises all aspects of a clinical trial. They’re usually helped by other doctors, nurses, and data managers who are part of the trial team.
A clinical trial coordinator ensures trials are conducted in a way that follows good clinical practice. They conduct the trial under the supervision of the principal investigator.
If you’re a volunteer who takes part in a trial, you’re a participant. Some trials are looking for patient volunteers (participants with a particular condition) and others are looking for healthy volunteers. You’ll need to be eligible (meet specific criteria) and sign an informed consent form before you can join.
Some trials require a caregiver to take part alongside the person they care for. We call those people ‘trial partners.’ Trial partners may be asked to sign an informed consent form before they take part in a clinical trial. Find out more about the role of a caregiver.
Where do clinical trials take place?
Nowadays, trial visits may take place in many different locations. Technology has also made it possible for some visits to take place virtually. Example of locations include: